Abstract
Background: Patients (pts) with higher-risk MDS have poor outcomes. Sabatolimab (MBG453) is a novel immunotherapy targeting T-cell immunoglobulin domain and mucin domain-3 (TIM-3), an immuno-myeloid regulator expressed on both immune and leukemic stem cells. Sabatolimab+HMA delivered durable responses in a Phase (Ph) Ib study in pts with high-risk/very high risk (HR/vHR)-MDS (Brunner AM, ASH 2021). We report disease characteristics from 2 ongoing randomized, double-blind, placebo-controlled studies of sabatolimab+HMA in higher-risk MDS (Ph II STIMULUS-MDS1 [NCT03946670] and Ph III STIMULUS-MDS2 [NCT04266301]) and compare different scoring systems (International Prognostic Scoring System [IPSS], revised IPSS [IPSS-R] and molecular IPSS [IPSS-M]) in this overall population.
Methods: We enrolled pts ≥18 years old with intermediate-risk (IR)/HR/vHR-MDS per IPSS-R not eligible for stem cell transplant or intensive chemotherapy. In STIMULUS-MDS1 (MDS1) only, pts with IR-MDS had to have ≥5% bone marrow (BM) blasts at baseline. Pts with chronic myelomonocytic leukemia-2 (CMML-2) were only eligible in STIMULUS-MDS2 (MDS2). Pts were randomized 1:1 to sabatolimab+HMA (azacitidine [AZA] or decitabine) or placebo+HMA in MDS1, and to sabatolimab+AZA or placebo+AZA in MDS2. Baseline mutations were evaluated by a targeted 37-gene next-generation sequencing panel on genomic DNA from BM mononuclear cells and/or peripheral blood (2% variant allele frequency sensitivity). No data were collected for del17/17p, TP53 loss-of-heterozygosity (LOH), BCORL1, GNB1, NF1, PPM1D, PRPF8, and MLL-PTD. IPSS-M was derived based on Bernard et al, 2022 (R package from https://github.com/papaemmelab/ipssm) using available clinical, cytogenetic, and mutation data. Given low prevalence of MLL-PTD, this alteration was considered nonmutated for all pts.
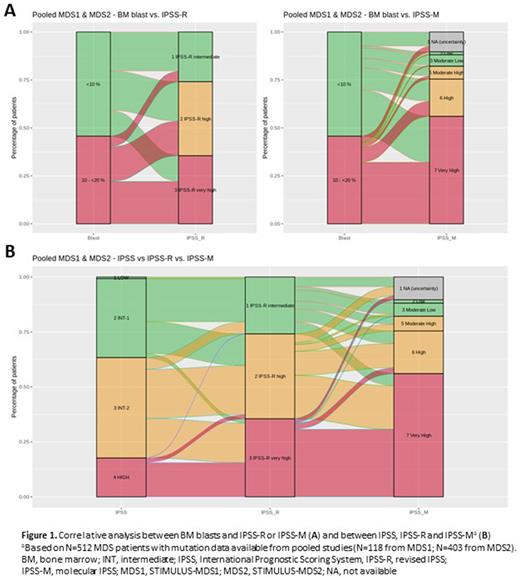
Results: In total, 657 pts were enrolled (MDS1, n=127; MDS2, n=530: MDS, n=492 and CMML-2, n=38). Further analyses focused on the 619 pts with MDS in MDS1 and MDS2. Of these, respectively, 87.4% and 76.8% were ≥65 years old; 67.7% and 65.2% were males. In MDS1, de novo disease was reported in 89.8% and secondary disease in 10.2% of pts; in MDS2 <1% had secondary disease. In MDS1, cytogenetic risk categories were very good/good in 40.9%, intermediate in 18.9%, and very poor/poor in 40.2% of pts; respective categories in MDS2 were 45.9%, 24.4%, and 29.7%. BM blast counts <10% were reported in 48.0% of pts in MDS1 and 58.1% in MDS2. Of pts enrolled in MDS1, 16.5%, 37.8%, and 45.7% were IR, HR, and vHR MDS per IPSS-R, respectively, and in MDS2 were 29.3%, 36.0% and 34.8%. The top 5 mutated genes in 118 pts with mutation testing in MDS1 were TP53 (34.7%), ASXL1 (33.9%), DNMT3A (27.1%), TET2 (25.4%), and RUNX1 (25.4%). The most frequently mutated genes in 403 pts with mutation testing in MDS2 were ASXL1 (38.7%), TET2 (28.3%), and RUNX1 (28.5%); a lower proportion of MDS2 pts vs MDS1 had mutations in TP53 (26.1%) and DNMT3A (17.4%). In MDS1, preliminary IPSS-M scores based on available data were low/moderate-low/moderate-high in 9.3%, high in 22.0%, and very high in 58.5% of pts (10.2% not available [uncertainty]). Respective IPSS-M scores for MDS2 were 15.6%, 18.6%, and 55.3% (10.4%). BM blasts 10-20% were associated with higher-risk categories, the majority being vHR IPSS-R and IPSS-M although these risk groups also included pts with <10% blasts (Figure 1A). Upstaging was observed from derived former IPSS criteria to IPSS-R. Furthermore, when comparing IPSS-R and IPSS-M, we observed that of pts with IR IPSS-R 22.2% and 21.5% were upstaged to HR and vHR IPSS-M respectively; 51.2% of pts with HR IPSS-R were upstaged to vHR IPSS-M; and 86.5% of pts with vHR IPSS-R remained vHR and 7.6% were downstaged to HR IPSS-M (Figure 1B).
Conclusions: Pts in MDS1 had poorer prognosis features (baseline blasts, cytogenetics and higher IPSS-R) compared with MDS2 study. This preliminary analysis is among the first in large, well-controlled trials to assess IPSS-M and shows higher-risk categories compared with IPSS-R. Until molecular testing is routine globally, pt selection for clinical trials is still dependent on IPSS-R. However, these results demonstrate that complete molecular and cytogenetic assessment at baseline will improve evaluation in clinical trials and provide useful information for treatment decisions. Further analyses are planned in the STIMULUS clinical trial program of sabatolimab.
Disclosures
Santini:Novartis, AbbVie, BMS, Gilead, Menarini, Geron, Otsuka: Honoraria. Platzbecker:Geron: Honoraria; BMS/Celgene: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Silence Therapeutics: Honoraria. Fenaux:Celgene/BMS: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Jazz: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Giagounidis:BMS: Honoraria. Miyazaki:Otsuka Pharmaceutical: Honoraria; Kyowa-Kirin: Honoraria; Pfizer: Honoraria; Bristol-Myers: Honoraria; Chugai: Honoraria; SyinBio: Honoraria; Dainippon-Sumitomo Pharma: Honoraria, Research Funding; Astellas: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Nippon Shinyaku: Honoraria; Takeda: Honoraria; Daiichi-Sankyo: Honoraria; Janssen Pharmaceutical: Honoraria; Celgene: Honoraria. Sekeres:Bristol Myers-Squibb: Membership on an entity's Board of Directors or advisory committees; Takeda/Millenium: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kurome: Membership on an entity's Board of Directors or advisory committees. Sanz:takeda: Honoraria; Janssen Pharmaceuticals, Inc.: Other: Teaching and Speaking; Celgene Corporation: Consultancy; Novartis Oncology: Consultancy; La Hoffman Roche Ltd.: Other: Advisor or review panel participant; Abbvie Pharmaceuticals: Other: Advisor or review panel participant; Helsinn: Honoraria, Other: Advisor or review panel participant; Takeda Pharmaceuticals Ltd: Other: Advisor or review panel participant. Van Hoef:Novartis Pharma AG: Current Employment. Ma:Novartis Pharmaceuticals Corporation: Current Employment. Hertle:Novartis Pharma AG: Current Employment. Marques Ramos:Novartis Pharma AG: Current Employment. Zeidan:Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal